Novel research finds genetic explanation for bone damage among survivors of childhood cancer
Summary:
Dr. Yaron Finkelstein, lead author of the study and Staff Physician in Emergency Medicine and Clinical Pharmacology and Toxicology and Senior Associate Scientist at SickKids explains how his work could improve health outcomes for paediatric survivors of ALL.
The majority of children diagnosed with acute lymphoblastic leukemia (ALL) will survive thanks to the success of chemotherapy and other cancer treatments. While these children survive the cancer, many will experience treatment-related complications that could lead to long-term health challenges.
In a new study published in Pediatric Blood & Cancer a team of researchers from The Hospital for Sick Children (SickKids), Einstein College of Medicine (led by Dr. Peter Cole) and Dana-Farber Cancer Institute/Boston Children's Hospital took a closer look at the genetic variations that may be contributing to one of the serious side effects of chemotherapy; bone toxicity including bone fractures and osteonecrosis (degeneration of bones due to lack of blood supply).
In this Science Show and Tell, Dr. Yaron Finkelstein, lead author of the study and Staff Physician in Emergency Medicine and Clinical Pharmacology and Toxicology and Senior Associate Scientist at SickKids explains how this work could improve health outcomes for paediatric survivors of ALL.
What did you and your team find?
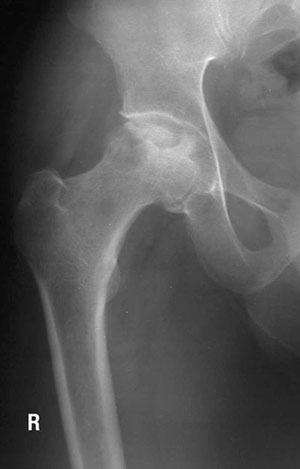
Bone fractures and osteonecrosis have been associated with exposure to common leukemia therapy drugs such as corticosteroids and methotrexate. Bone toxicity is an extremely debilitating condition that often leads to lifelong pain, shortened limbs, has difficult recovery and may lead to impaired quality of life. Our team wanted to explore if there are any genetic variants that make some children more at risk for bone damage including fractures and osteonecrosis.
Our findings are significant, as we found that 20 per cent of the 615 study’s participants had a genetic variant in the gene for thymidylate synthase that was associated with increased risk of bone fractures and osteonecrosis. We also found that kids who were diagnosed with leukemia at age 10 or older were more at risk for bone fractures, while those who were younger than 10 years old when they had leukemia were more at risk for osteonecrosis. This is a novel observation.
Describe the significance of the study’s findings?
We studied genetic variations within genes related to the drugs most frequently implicated in causing bone toxicity among children treated for ALL. We found a specific variant in the Thymidylate Synthase gene, which is associated with the metabolism of methotrexate, a key chemotherapy drug. Our findings could guide clinical care suggesting that kids and teens with leukemia, who carry this variant in the Thymidylate Synthase gene should be more closely monitored for potential bone toxicity during therapy.
Finkelstein is also Professor in the Departments of Paediatrics and Pharmacology and Toxicology at the University of Toronto.
This work was supported in part by the Michael Garil Fund for Leukemia Research and a St. Baldrick’s Foundation Supportive Care Research grant.
This project is an example of how SickKids is contributing to making Ontario Healthier, Wealthier and Smarter. www.healthierwealthiersmarter.ca.

